Thoughtful Design
This novel furosemide combination product received Tentative Approval from the FDA in October 2024. It is not approved for sale.
The Infusor was specifically designed for administering furosemide to patients with heart failure, with careful consideration given to the needs of patients, providers, payors, and the environment.
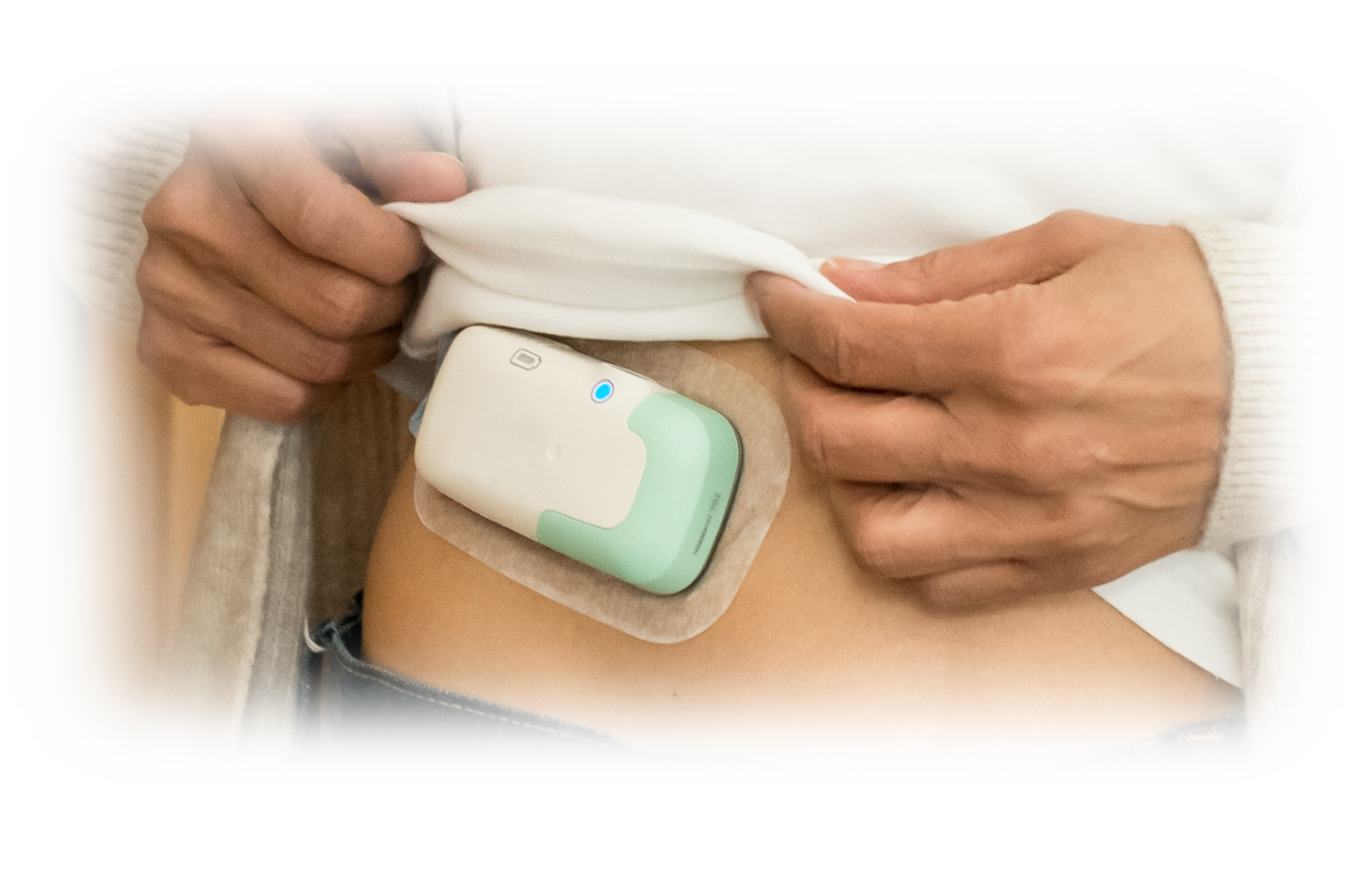
Designing for the Patient – Small is Better
First we wanted the Infursor to be small and light for comfort during use and so we could use the least aggressive medical tape. This may sound trivial, but the abdominal skin of the elderly is fragile and removing more aggressive tape may hurt and cause bruising or skin damage.
Designing for the Provider – Fitting how Providers wish to Treat
Worsening heart failure and decongestion follow established clinical workflows. We designed our new treatment to integrate seamlessly into how clinicians already manage patients with heart failure.
A common scenario involves a patient with congestion being seen in an outpatient clinic, urgent care center, or even the emergency department. If there’s no immediate need for hospital admission, clinicians prefer to start treatment right away.
To support this, Lasix ONYU will have a 24-month shelf life and, following approval, will be distributed by leading pharmaceutical suppliers. This ensures availability in hospitals and clinics to be available onsite when patients need it.
Our user-friendly design enables clinicians to train the patient, prepare and place the device, and send the patient home. Once home, the patient simply presses a button to begin therapy. The Lasix ONYU Infusor provides a 7-hour window between device placement and the start of treatment—ample time for nearly all patients to reach the comfort of their home.


Designing for the Payor – Affordable thanks to Reuse of Electronics
Electronics are inherently expensive due to the scarcity and high cost of the materials used. Electronic devices like the Lasix ONYU Infusor contain essential components such as a motor, controllers (chips), LEDs, and a battery. The electronic components of the Lasix ONYU Infusor are designed to last for 48 treatments. The Infusor is charged in about five minutes between treatments.
Importantly, the disposable part of the Infusor for each treatment is far less expensive than a fully electronic device, keeping overall costs low. This design benefits both patients and payors by making the treatment more affordable. Additionally, the simplified design enables easier automated production, further driving down costs. We have already developed a high-capacity robotic manufacturing line, ready to begin production upon approval.
Designing for the Planet – Avoiding Electronic Medical Waste
Medical waste is incinerated and then end-up in landfills. Our two component design assures that the electronic components and the battery are not part of the medical waste stream. First the electronics can be used for 48 treatments and secondly the electronics can be recycled. This avoids all electronic components in medical waste.
We also wanted to avoid chemical sterilization. Medical devices with electronic components are commonly sterilized with chemicals, most commonly ethyleneoxide, a known carcinogen.

