FDA Approved

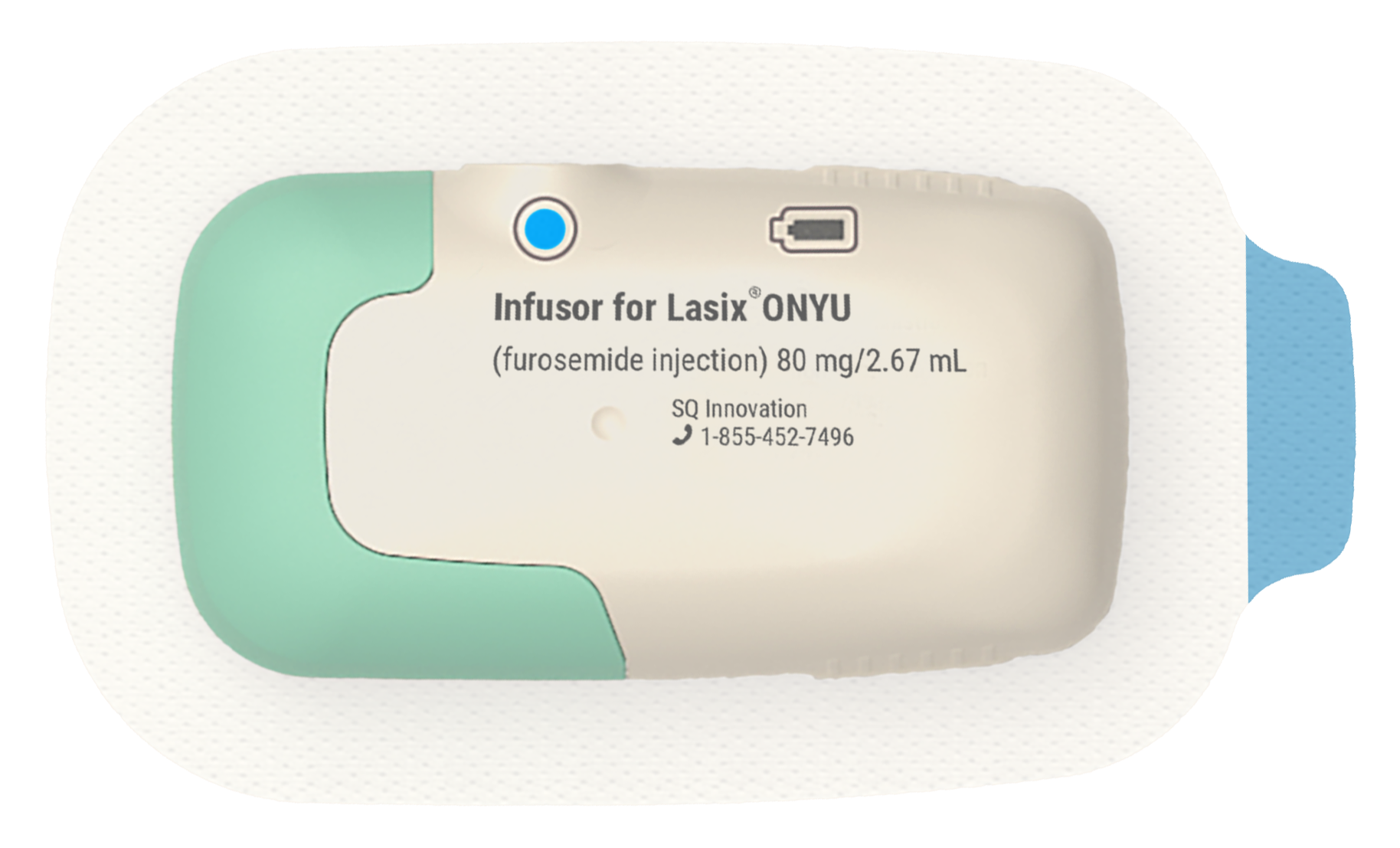
For Further Information About Lasix® ONYU, visit www.lasix-onyu.com
For Information About Pricing and Availability, See Under FAQ on this Site.
Preparing for US Launch
We are finalizing preparations for the U.S. commercial launch. The drug and device components are already manufactured, with labeling and packaging now underway.
In parallel, we are collaborating with leading hospital systems to develop innovative workflows that enable more patients to receive treatment at home. If you’d like to be part of this effort, please reach out so we can explore working together.
Home is Where my Bathroom is! ™

